Applications
 |
Applications |
| In the following, various features and experimental applications of the pZ expression system are presented: 1 IPTG-inducible expression of recombinant proteins 2 Tetracycline-inducible expression of recombinant proteins 2.1 Using Tetracycline, Anhydrotetracycline or Doxycycline as inducer 3 Graded induction of gene expression 3.1 Graded induction of genes controlled by PA1lacO-1 3.2 Graded induction of genes controlled by Plac/ara-1 3.3 Graded induction of genes controlled by PLtetO-1 and PN25tetO-1 4 Independent simultaneous and non-simultaneous expression of two gene activities 4.1 Coexpression of S. cerevisiae alpha-Glucosidase and E. coli chaperones DnaK, DnaJ: Influence on solubility and activity of alpha-Glucosidase 4.2 Simultaneous and non-simultaneous expression of two interacting MSP-1 subunits (p120 and p85) from P. falciparum: Influence on expression rates 5 Expression of toxic gene products: Production of restriction enzyme Cfr9I |
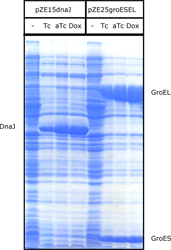
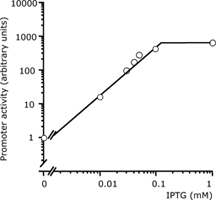
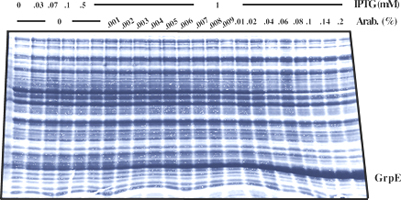
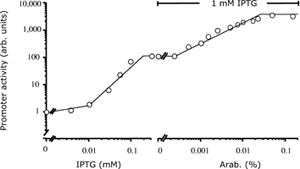
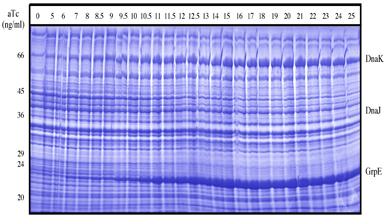
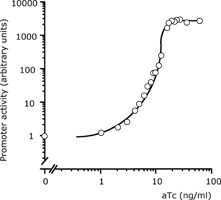
| 4 Independent simultaneous and non-simultaneous expression of two gene activities 4.1 Coexpression of S. cerevisiae alpha-Glucosidase and E. coli chaperones DnaK, DnaJ: Influence on solubility and activity of alpha-Glucosidase |
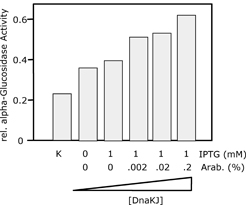
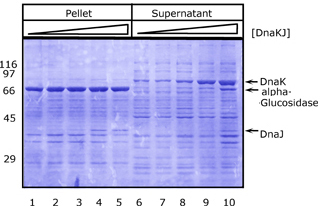
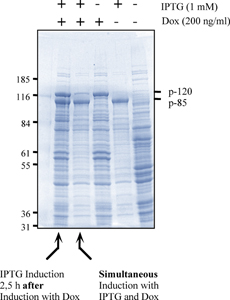
| 4.2 Simultaneous and non-simultaneous expression of two interacting MSP-1 subunits (p120 and p85) from P. falciparum: Influence on expression rates |
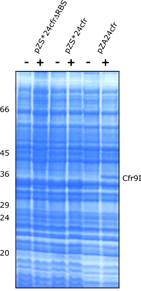
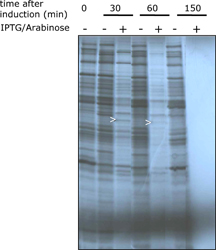
| Experimental setup: • Cultures of DH5alphaZ1 harbouring pZS*24cfr (- RBS), pZS*24cfr (+RBS), or pZA24cfr (+RBS) were grown to mid log phase in LB medium (Notes: Cfr9I was expressed without its cognate Methyltransferase M.Cfr9I. The presence of an efficient RBS (+ RBS) increased expression levels around 10 fold). • Gene expression was induced by 1mM IPTG and 0.2% (w/v) Arabinose for 2.5 hrs. • Proteins were separated by SDS-PAGE and stained with Coomassie (A). • Pulse-chase experiment: • A culture of DH5alphaZ1 harbouring pZA24cfr (+RBS) was grown to early log phase in minimal medium. • After splitting the culture in aliquots gene expression was induced by 1mM IPTG and 0.2% (w/v) Arabinose. • Newly synthesized proteins were labeled at various time points by the addition of 35S-Methionine • Proteins were separated by SDS-PAGE and made visible by autoradiography (B) Results: • Cfr9I can be expressed to 2-3% of total cellular protein if encoded on a plasmid harbouring a p15A ori. • No viable cells were detectable in any culture 20 min after induction of cfr9I (data not shown). • No mucoid bacterial growth or plasmid instabilities were observed under conditions of repression (data not shown). • Expression of cfr9I leads to a decrease in protein production over time. • 150 min after induction no protein systhesis can be detected (Note: expression of cfr9I causes immediate cell death). |